- CRMO
-
고객과 함께 미래를 열어가는 WELL GREEN
아름답고 건강한 변화와의 약속
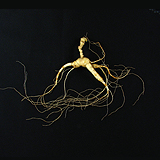
인삼(산삼)
- 학명 : Panax ginseng C.A.Meyer
- 원산지 : 한국, 중국, 러시아
- 지표성분 : Ginsenoside 등
- 주요효능 : 면역, 혈행개선, 간보호, 항당뇨

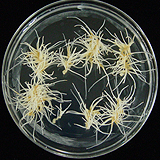
| 세포주 기원 | 뿌리 |

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-PG-C1~6, WG-PG-A1~9 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 특허 |
|
|
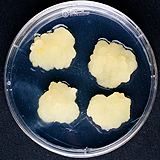
| 세포주 기원 | 종자 |

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-MC-C1, WG-MC-A1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
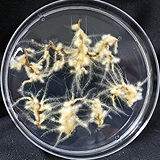
| 세포주 기원 | 잎 |

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-RA-A1, WG-RA-P1 | |
| 배양환경 | 명/암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

병풀
- 학명 : Centella asiatica (L.) Urb.
- 원산지 : 인도, 스리랑카, 남아프리카 등
- 지표성분 : Asiaticoside, Asiatic acid 등
- 주요효능 : 피부 상처, 화상, 궤양 등

| 세포주 기원 | 뿌리, 잎 |
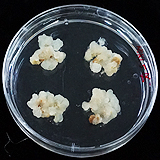
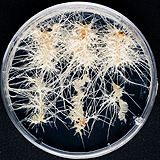
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-CA-C1, WG-CA-A1-5, WG-CA_H1-8 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

꽃치자
- 학명 : Gardenia jasminoides var. radicans (Thunb.) Makino
- 원산지 : 중국
- 지표성분 : Gardenoside, geniposide 등
- 주요효능 : 청열, 양혈, 해독 등

| 세포주 기원 | 꽃 |
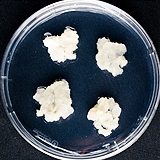
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-GJ-C1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
![웰그린 서양고추나물[세인트 존스 워터] 사진](/images/cont/c4/c4_1img6.jpg)
서양고추나물[세인트 존스 워터]
- 학명 : Hypericum perforatum L.
- 원산지 : 유럽
- 지표성분 : Hypericin, Hyperforin 등
- 주요효능 : 항산화, 진정제, 정신질환 등

| 세포주 기원 | 잎, 뿌리 |
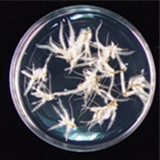
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-HP-A1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 세포주 기원 | 종자 |
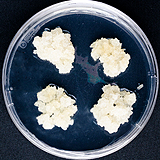
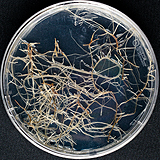
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-GM-C1~9, WG-GM-A1~8 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

담배
- 학명 : Nicotiana tabacum L.
- 원산지 : 남아메리카열대
- 지표성분 : Alkaloid, rutin, cycloartenol 등
- 주요효능 : 소화불량, 통증 완화 등

| 세포주 기원 | 종자 |

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-NT-C1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 세포주 기원 | 잎 |
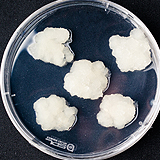
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-CS-C1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 세포주 기원 | 잎 |
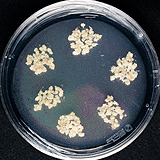
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-CS-C1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

수정목
- 학명 : Damnacanthus major Siebold & Zucc.
- 원산지 : 한국, 일본
- 지표성분 : Anthraquinone, Damnacanthal 등
- 주요효능 : 거풍(祛風), 이습(利濕) 등

| 세포주 기원 | 잎, 꽃 |
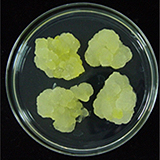
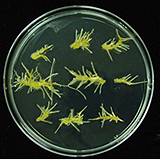
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-DM-Cl, WG-DM-A1, WG-DM-P1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 세포주 기원 | 뿌리 |


|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-PM-A1-5, WG-PM-H1-2, WG-PM-P1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

덴드로비움(철피석곡)
- 학명 : Dendrobium candidum Wall ex Lindl
- 원산지 : 한국, 일본, 중국, 열대, 호주 등
- 지표성분 :Erianin 등
- 주요효능 : 기관지, 해열, 시력 등

| 세포주 기원 | 잎, 꽃 |


|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-DN-L1-2, WG-DN-P1-2 | |
| 배양환경 | 명배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

홍경천(바위돌꽃)
- 학명 : Rhodiola rosea L.
- 원산지 : 한국(북부), 일본, 시베이라 등
- 지표성분 : Rosavin, Rosarin 등
- 주요효능 : 강장제, 항당뇨, 관절염 치료 등

| 세포주 기원 | 잎 |

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-RS-L1, WG-RS-P1 | |
| 배양환경 | 명배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 세포주 기원 | 잎, 줄기 |


|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-AF-R1, WG-AF-P1 | |
| 배양환경 | 명배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

에키네시아
- 학명 : Echinacea purpurea (L.) Moench
- 원산지 : 북미, 동부 아메리카
- 지표성분 : Cichoric acid, Echinacoside 등
- 주요효능 : 면역, 항알러지 등

| 세포주 기원 | 종자, 잎, 뿌리 |


|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-EAN-A1, WG-EPA-A1, WG-EPU-A1, WG-EAN-P1, WG-EPA-P1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 특허 |
|
|
| 세포주 기원 | 꽃, 잎 |
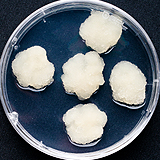
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-PC-C1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

할미꽃
- 학명 : Pulsatilla koreana (Yabe ex Nakai) Nakai ex Mori
- 원산지 : 한국
- 지표성분 : Hederacolchiside Pulsatilla saponin 등
- 주요효능 : 치매, 항암, 말라리아 등

| 세포주 기원 | 종자 |
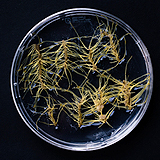
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-PK-A1-6, WG-PK-P1 | |
| 배양환경 | 명·암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 세포주 기원 | 종자 |
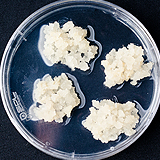

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-CJ-C1-5, WG-CJ-A1-5 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 페트리디쉬 | |
| 논문 |
|
|

펠라르고늄
- 학명 : Pelargonium sidoides DC.
- 원산지 : 남아프리카
- 지표성분 : coumarins, penolic acids 등
- 주요효능 : 감기, 인플루엔자 치료제 등

| 세포주 기원 | 종자 |
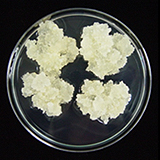
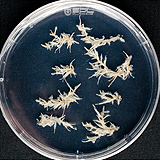
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-PS-C1, WG-PS-A1, WG-PS-P1 | |
| 배양환경 | 명·암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|
| 세포주 기원 | 종자 |

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-RU-P1 | |
| 배양환경 | 명·암배양, 22±1℃ | |
| 공정개발단계 | 생물반응기 | |
| 논문 |
|
|

미선나무
- 학명 : Abeliophyllum distichum Nakai
- 원산지 : 한국
- 지표성분 : acteoside, isoacteoside 등
- 주요효능 : 항산화, 미백, 아토피 개선 등

| 세포주 기원 | 꽃 |
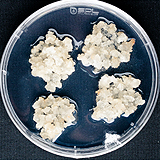
|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-AD-C1 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 페트리디쉬 | |
| 논문 |
|
|

에델바이스
- 학명 : Leontopodium alpinum Nakai
- 원산지 : 유럽, 남아메리카 고산지대
- 지표성분 : phenylpropanoid 등
- 주요효능 : 민감성 피부, 스트레스 완화 등

| 세포주 기원 | 잎 |


|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-LA-C1, WG-LA-A1, WG-LA-P1 | |
| 배양환경 | 명·암배양, 22±1℃ | |
| 공정개발단계 | 페트리디쉬 | |
| 논문 |
|
|
| 세포주 기원 | 꽃 |

|
|---|---|---|
| 세포주 보유현황 |
|
|
| 세포주 자원번호(No.) | WG-RH-C1-3 | |
| 배양환경 | 암배양, 22±1℃ | |
| 공정개발단계 | 페트리디쉬 | |
| 논문 |
|
|












